Company profile
- Name
- Immuno Probe Co. Ltd.
- Established
- November 7, 1986
- Capital
- 10,000,000 yen
- President
- Hiroshi Nomura
- Employees
- 20
- Head Office
- 1331-3, Kamagata, Ranzan-machi, Hiki-gun, Saitama, 355-0225, JAPAN
- Contact
- TEL: +81-493-62-6923
FAX: +81-493-62-9477
info@immuno-probe.com
Company history
- 1986
- Establish Immuno Probe Co., Ltd. in Shiga, Ranzan-machi, Hiki-gun, Saitama for the purpose of preparation of polyclonal antibodies for latex-immune assay
- 1988
- Start preparation of monoclonal antibodies
- 1944
- Relocate the facilities in Kamagata, Ranzan-machi, Hiki-gun, Saitama, and start safety testing and pharmacological test
- 2006
- Obtain ISO 13485 certification
- 2008
- Obtain Medicinal Chemical Manufacturer License
- 2011
- Submission to American Association for Clinical Chemistry (AACC) Join government-financed aid project for cooperation between hospitals and private companies for one year
Join government-financed aid project for medical device development /improvement for three years - 2012
- Start contracted manufacture for in-vitro diagnostic drug medicine Join the Saitama prefecture-project of medical manufacturing industry for three years
- 2013
- Conduct Saitama prefecture-financed aid project for next-generation industry entry for one year Put up a display booth in Medica (Germany)
- 2014
- Obtain Second Class Manufacturing and Distribution License
Conduct manufacturing, trade, and service innovation business for small companies for one year - 2015
- Launch in-vitro diagnostic medicine IP line Duo 「Noro/Rota」
Quality Policy
- 1.An approach to quality improvement and
customer satisfaction improvement regarding our product lines and their service - 2.Validate the effectiveness of quality
management system periodically, and improve it continuously
Mission
~From raw materials to in-vitro diagnostic~
We aim for the following :Preparation of better antibodies
Development of better in-vitro diagnostic
Introduction of innovative technology
Characteristic manufacturing technology
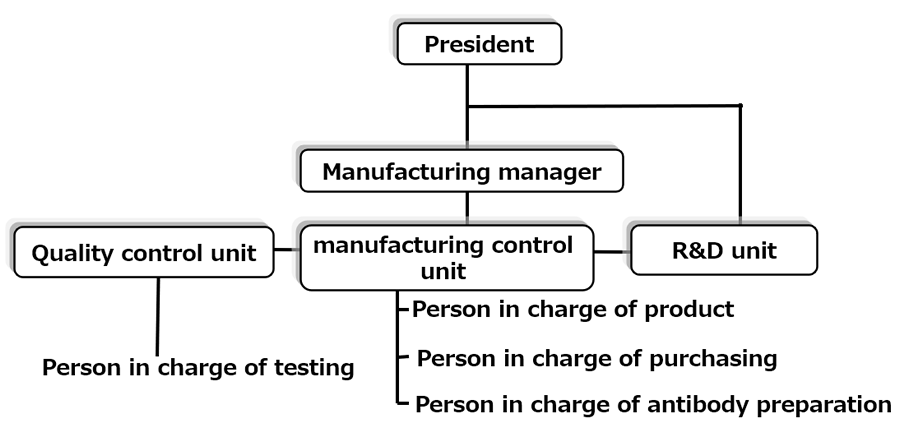
Company Structure
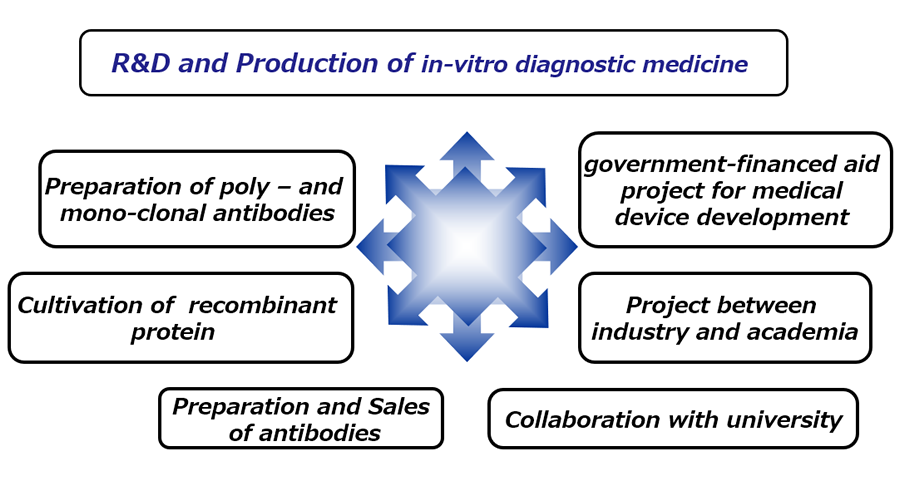
Business lineup
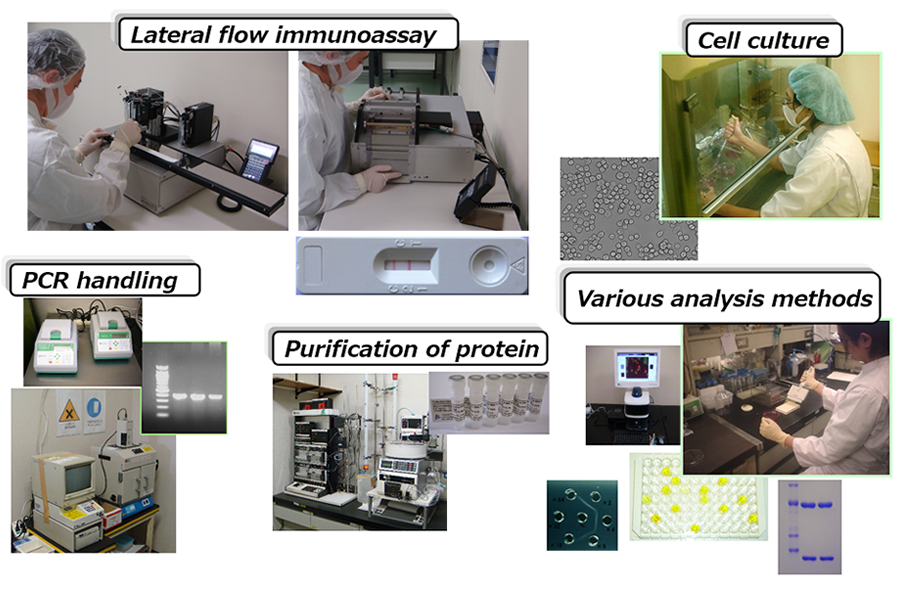
Technology
Product line
in-vitro diagnostic medicine:
Lateral flow immunoassay
- ・Noro antigen detection kit
- ・Mycobacterium antigen detection kit
- ・Simultaneous detection kit of Noro/Rota antigens
Supply of poly- and mono-clonal antibodies

